NCERT Solutions for Class 12th Chemistry Chapter 15: Polymers
Question 15.1:
Explain the terms polymer and monomer.
Answer
Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (103 − 107 u). In a polymer, various monomer units are joined by strong covalent bonds. Polymers can be natural as well as synthetic. Polythene, rubber, and nylon 6, 6 are examples of polymers.
Monomers are simple, reactive molecules that combine with each other in large numbers through covalent bonds to give rise to polymers. For example, ethene, propene, styrene, vinyl chloride.
Question 15.2:
What are natural and synthetic polymers? Give two examples of each type.
Answer
Natural polymers are polymers that are found in nature. They are formed by plants and animals. Examples include protein, cellulose, starch, etc. Synthetic polymers are polymers made by human beings. Examples include plastic (polythene), synthetic fibres (nylon 6, 6), synthetic rubbers (Buna − S).
Question 15.3:
Distinguish between the terms homopolymer and copolymer and give an example of each.
Answer
| Homopolymer |
Copolymer |
| The polymers that are formed by the polymerization of a single monomer are known as homopolymers. In other words, the repeating units of homopolymers are derived only from one monomer. For example, polythene is a homopolymer of ethane. |
The polymers whose repeating units are derived from two types of monomers are known as copolymers. For example, Buna−S is a copolymer of 1, 3-butadiene and styrene. |
Question 15.4:
How do you explain the functionality of a monomer?
Answer
The functionality of a monomer is the number of binding sites that is/are present in that monomer.
For example, the functionality of monomers such as ethene and propene is one and that of 1, 3-butadiene and adipic acid is two.
Question 15.5:
Define the term polymerisation.
Answer
Polymerization is the process of forming high molecular mass (10^3 − 10^7 u) macromolecules, which consist of repeating structural units derived from monomers. In a polymer, various monomer units are joined by strong covalent bonds.

Question 15.7:
In which classes, the polymers are classified on the basis of molecular forces?
Answer
On the basis of magnitude of intermolecular forces present in polymers, they are classified into the following groups:
(i) Elastomers
(ii) Fibres
(iii) Thermoplastic polymers
(iv) Thermosetting polymers
Question 15.8:
How can you differentiate between addition and condensation polymerisation?
Answer
Addition polymerization is the process of repeated addition of monomers, possessing double or triple bonds to form polymers. For example, polythene is formed by addition polymerization of ethene.
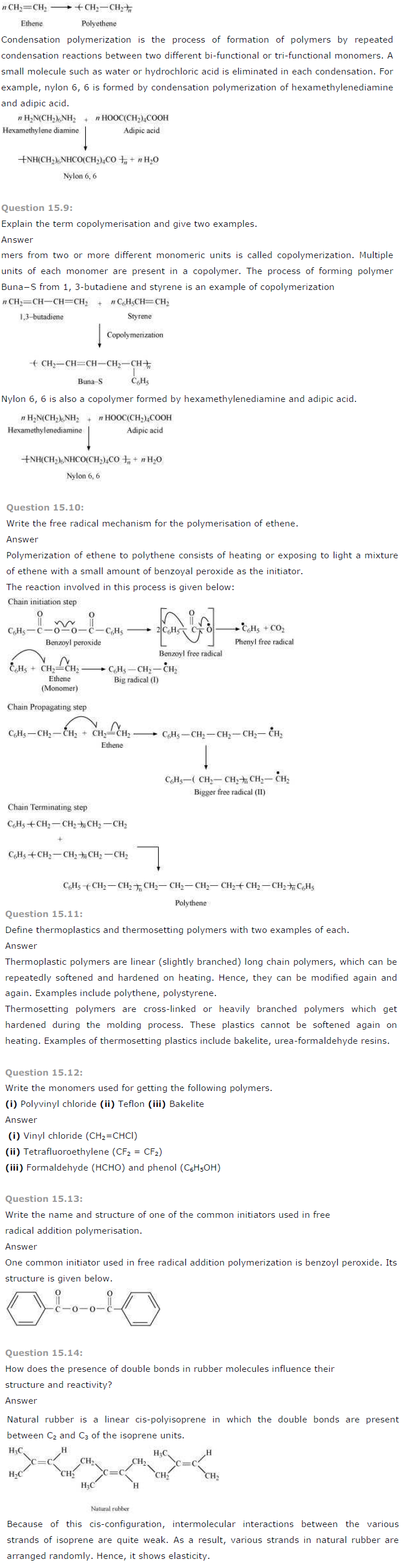
Question 15.15:
Discuss the main purpose of vulcanisation of rubber.
Answer
Natural rubber though useful has some problems associated with its use. These limitations are discussed below:
1. Natural rubber is quite soft and sticky at room temperature. At elevated temperatures (> 335 K), it becomes even softer. At low temperatures (< 283 K), it becomes brittle.
Thus, to maintain its elasticity, natural rubber is generally used in the temperature range of 283 K-335 K.
2. It has the capacity to absorb large amounts of water.
3. It has low tensile strength and low resistance to abrasion.
4. It is soluble in non-polar solvents.
5. It is easily attacked by oxidizing agents.
Vulcanization of natural rubber is done to improve upon all these properties. In this process, a mixture of raw rubber with sulphur and appropriate additive is heated at a temperature range between 373 K and 415 K.
Question 15.16:
What are the monomeric repeating units of Nylon-6 and Nylon-6, 6?
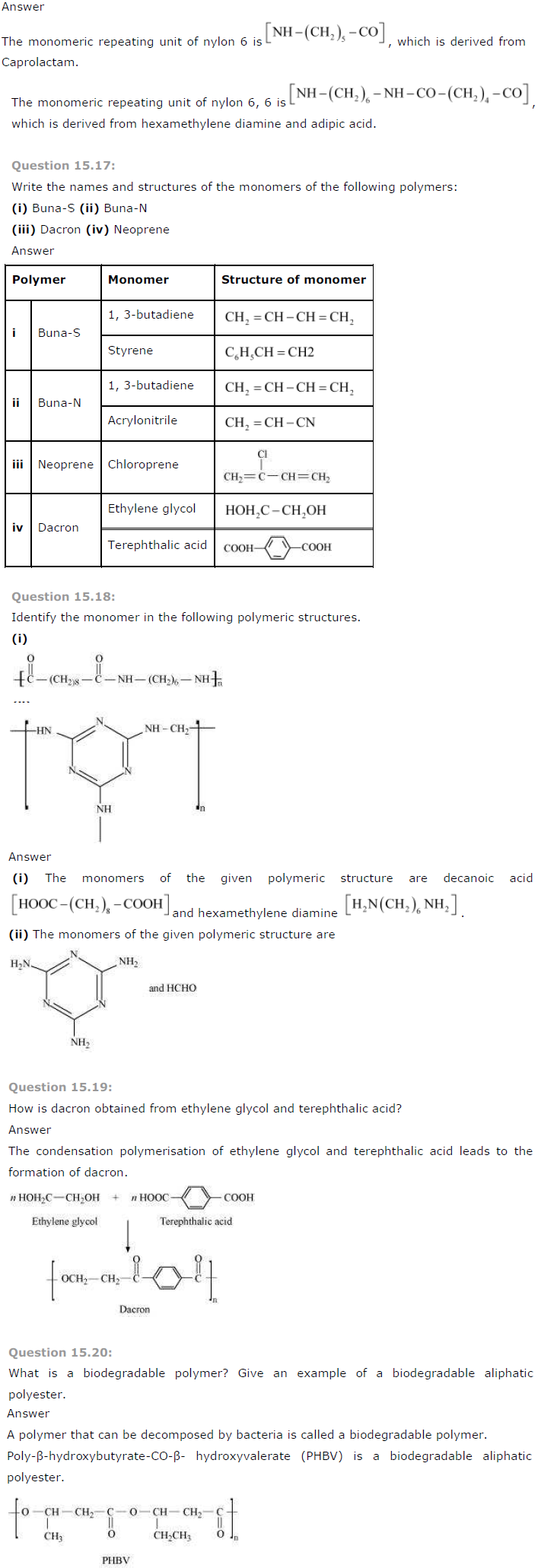
Question 15.1:
What are polymers?
Answer
Polymers are high molecular mass macromolecules, which consist of repeating structural units derived from monomers. Polymers have a high molecular mass (103 − 107 u). In a polymer, various monomer units are joined by strong covalent bonds. These polymers can be natural as well as synthetic. Polythene, rubber, and nylon 6, 6 are examples of polymers.
Question 15.2:
How are polymers classified on the basis of structure?
Answer
Polymers are classified on the basis of structure as follows:
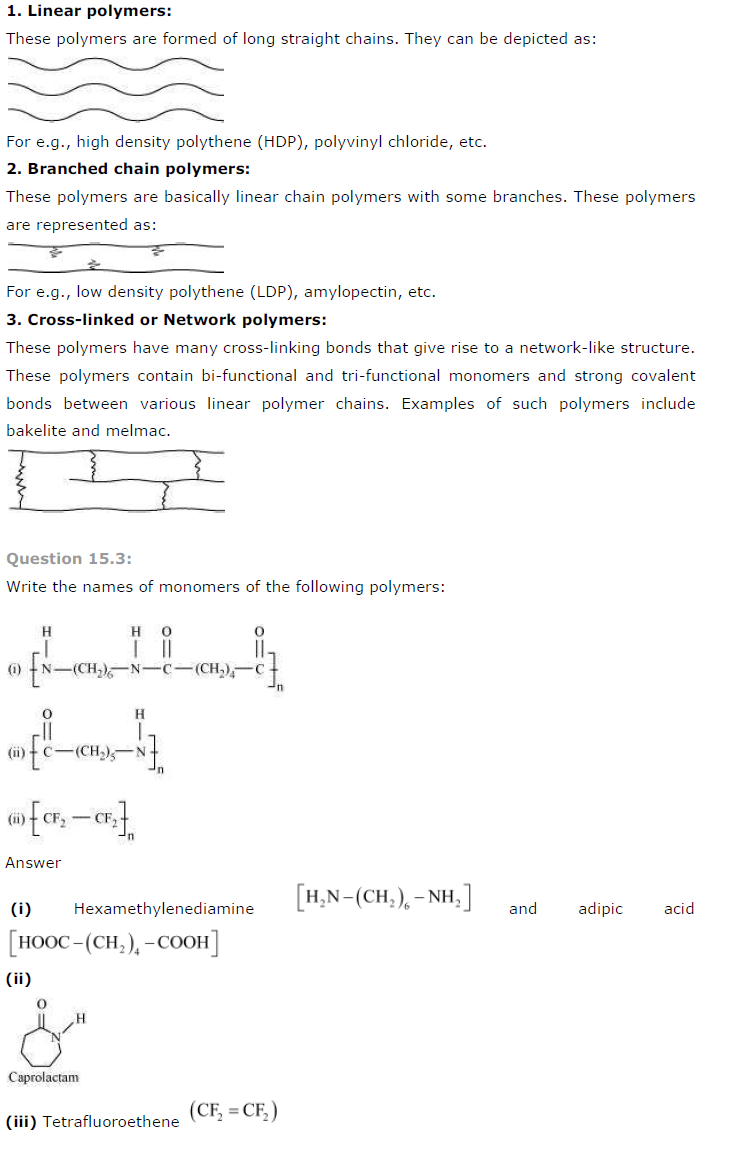
Question 15.4:
Classify the following as addition and condensation polymers: Terylene, Bakelite,
Polyvinyl chloride, Polythene.
Answer
Addition polymers:
Polyvinyl chloride, polythene
Condensation polymers:
Terylene, bakelite
Question 15.5:
Explain the difference between Buna-N and Buna-S.
Answer
Buna − N is a copolymer of 1, 3−butadiene and acrylonitrile.
Buna − S is a copolymer of 1, 3−butadiene and styrene.
Question 15.6:
Arrange the following polymers in increasing order of their intermolecular forces.
(i) Nylon 6, 6, Buna-S, Polythene.
(ii) Nylon 6, Neoprene, Polyvinyl chloride.
Answer
Different types of polymers have different intermolecular forces of attraction. Elastomers or rubbers have the weakest while fibres have the strongest intermolecular forces of attraction. Plastics have intermediate intermolecular forces of attraction. Hence, the increasing order of the intermolecular forces of the given polymers is as follows:
(i) Buna − S < polythene < Nylon 6, 6
(ii) Neoprene < polyvinyl chloride < Nylon 6
Go Back to NCERT Solutions Chemistry Page Physics Biology Maths
To start your test
register with us:
To start
your test, registered user must login with their User Name & Password: